Research into Silver Nanoparticles
8 déc 2019

Silver Nanoparticles are particles of silver between 1 nm and 100 nm in diameter. Due to the large ratio of surface-to-bulk silver atoms, they may also contain a large percentage of silver oxide.
Applications
Silver nanoparticles have found a wide range of applications including their use as catalysts, as optical sensors of zeptomole (10−21) concentrations, in textile engineering, in electronics, in optics, as anti-reflection coatings, and most importantly in the medical field as a bactericidal and therapeutic agent. Silver is used in the formulation of dental resin composites, in coatings of medical devices, as a bactericidal coating in water filters, as an antimicrobial agent in air sanitizer sprays, pillows, respirators, socks, keyboards, detergents, soaps, shampoos, toothpastes, washing machines and many other consumer products, in bone cement and in many wound dressings.1,2
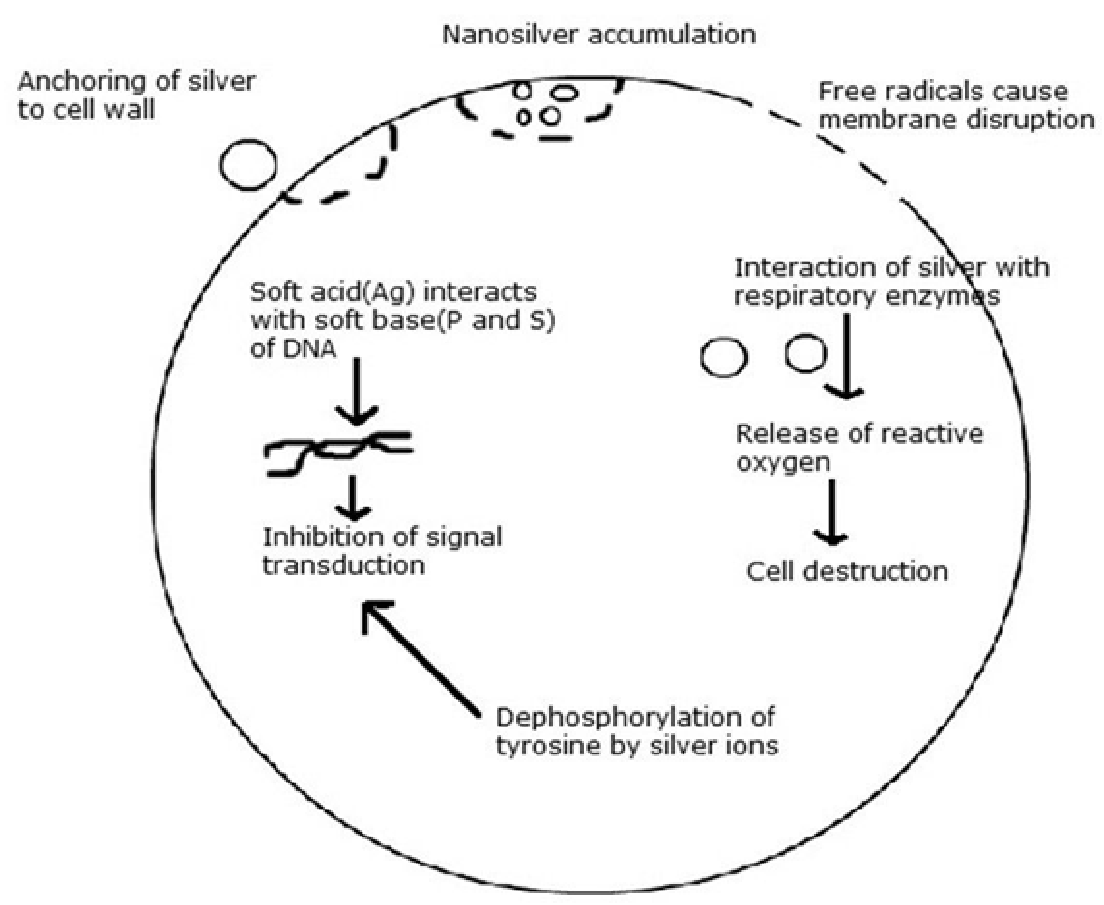
Silver nanoparticles provide a huge increase in the surface area available for the continuous release of a low level of silver ions to provide protection against bacteria. The mechanism(s) for this antibacterial action is not fully known. It has been suggested that silver nanoparticles can cause cell lysis or inhibit cell transduction.3,4 There are various mechanisms involved in cell lysis and growth inhibition as indicated in figure 1.
Synthesis
There are many ways to synthesize silver nanoparticles including physical, chemical, and biological methods. Most procedures involve the reduction of silver ions in solution; techniques include chemical reduction with borohydride, electro-reduction in the presence of polyethylene glycol, ultrasound to induce sonochemical reduction of an aqueous silver solution in an atmosphere of argon-hydrogen. Greener biological methods, using microbes, plants or fungi5,6 have also become popular.
Toxicity
The wide-spread use of silver nanoparticles has led to concerns about their toxicity which has been reported to cause various environmental and health problems.7,8
Recent Research
Silver nanoparticles remain an area of considerable research interest. Extensive studies have been carried out on the mechanisms of their bactericidal role. Carlo Brabante’s team at the University of Venice have characterised silver release from different types of dressing used in burns care.9,10 In this work and other research into silver nanoparticles it is essential to minimise blanks and experimental artefacts by specifying ultrapure water. Water from an ELGA Purelab Chorus system was used throughout.
A number of researchers have investigated green techniques for preparing silver nanoparticles. Typical examples are Thakur et al.11 and Faried et al.12. Thakur used photosynthesis at room temperature with walnut bark in a pure-water medium (ELGA Purelab flex) to generate silver nanoparticles with antibacterial properties. Transmission Electron Micrographs showed the presence of twinned nanoparticles. Faried used ultrasonics in a sodium alginate media prepared in purified water (ELGA Purelab).
The role of silver nanoparticles to enhance analytical techniques has been described by several authors. Yang OU and co-workers12 and Botto13 used them for surface-enhanced Raman scattering, while Bottger et al14 used them to sample air-borne mercury before its determination by total reflection x-ray fluorescence. In all cases ultrapure water from ELGA Purelab flex units, with their high specification water and convenient delivery, was used to prepare all working solutions.
References
- The bactericidal effect of silver nanoparticles. Morones. JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramirez JT, Yacaman MJ, Nanotechnology 2005, 16: 2346–2353.
- Antimicrobial effects of silver nanoparticles. Kim JS, Kuk E, Yu K, Kim JH, Park SJ, Lee HJ, Kim SH, Park YK, Park YH, Hwang C-Y, Kim YK, Lee YS, Jeong DH, Cho MH Nanomedicine 2007, 3: 95–101.
- A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. Feng QL, Wu J, Chen GQ, Cui FZ, Kim TN, Kim JO: J. Biomed. Mater. Res. 2008, 52: 662–668.
- Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. Sondi I, Salopek-Sondi B, J. Colloid Interface Sci. 2004, 275: 177–182.
- Biosynthesis of nanoparticles: technological concepts and future applications. Mohanpuria P, Rana KN, Yadav SK, J. Nanopart. Res. 2008, 10: 5 07–517.
- Fungus mediated synthesis of silver nanoparticles and their immobilization in the mycelial matrix: a novel biological approach to nanoparticle synthesis. Mukherjee P, Ahmad A, Mandal D, Senapati S, Sainkar SR, Khan MI, Parischa R, Ajaykumar PV, Alam M, Kumar R, Sastry M. Nano Lett. 2001, 1: 515–519.
- Silver or silver nanoparticles: a hazardous threat to the environment and human health? Panyala NR, Pena-Mendez EM, Havel J, J. Appl. Biomed. 2008, 6: 117–129.
- Subchronic oral toxicity of silver nanoparticles. Kim YS, Song MY, Park JD, Song KS, Ryu HR, Chung YH, Chang HK, Lee JH, Oh KH, Kelman BJ, Hwang IK, Yu IJ, Part. Fibre Toxicol. 2010, 7: 20.
- Characterization and evaluation of silver release from four different dressings used in burns care C. Rigo, M. Roman, I. Munivrana, V. Indigni, B. Azzena, Carlo Barbante, W. R.L.Cairns, Burns Volume 38, Issue 8, December 2012, 1131-114
- Hydrodynamic chromatography coupled to single-particle ICP-MS for the simultaneous characterization of AgNPs and determination of dissolved Ag in plasma and blood of burn patients. M. Roman, C. Rigo, H. Castillo-Michel, I. Munivrana, V. Vindigni, I. Mičetić, F. Benetti, L. Manodori and W. R. L. Cairns, A nalytical and Bioanalytical. Chemistry, July 2016, 408 (19) 5109–5124
- Phytosynthesis of Silver Nanoparticles Using Walnut (Juglans regia) Bark with Characterization of the Antibacterial Activity against Streptococcus mutans.N. Thakur, V. G.Gaikar, D. Sen, S. Mazumder & N. S.Pandita, Analytical Letters Volume 50, 2017 - Issue 4
- Green synthesis of silver nanoparticles in biopolymer stabilizer and their application as antibacterial efficacy. M. Faried, K. Shameli, M. Miyake, H. Hara, and N. Bahiyah Ahmad, AIP Conference Proceedings 1788, 030124 (2017)
- In-Situ Immobilization of Silver Nanoparticles on Self-Assembled Honeycomb-Patterned Films Enables Surface-Enhanced Raman Scattering (SERS) Substrates. Yang Ou, Li-Yang Wang, Liang-Wei Zhu, Ling- Shu Wan, Zhi -Kang Xu J. Phys. Chem. C 201411821, 11478-11484
- Direct analysis of anthraquinone dyed textiles by Surface Enhanced Raman Spectroscopy and Ag nanoparticles obtained by pulsed laser ablation. A Botto, B Campanella, I Degano, S Legnaioli, G Lorenzetti, Stefano Pagnotta, F Poggialini, V Palleschi, The European Physical Journal Plus, August 2019, 134:414
- Evaluating internal standards for the determination of gas phase mercury using silver nanoparticle assisted total reflection X-ray fluorescence. S Böttger, IMB Tyssebotn, W Jansen, UEA Fittschen, Spectrochimica Acta Part: Atomic Spectroscopy, Sept. 2018, 147, 93-99
Dr Paul Whitehead
After a BA in Chemistry at Oxford University, Paul focused his career on industrial applications of chemistry. He was awarded a PhD at Imperial College, London for developing a microwave-induced-plasma detector for gas chromatography. He spent the first half of his career managing the analytical support team at the Johnson Matthey Research/Technology Centre,specialising in the determination of precious metals and characterising applications such as car-exhaust catalysts and fuel cells. Subsequently, as Laboratory Manager in R&D for ELGA LabWater, he has been involved in introducing and developing the latest water purification technologies. He now acts as a consultant for ELGA.
